May 6, 2013 (revised 12-23-2014) — This article discusses the evolution of the labeling of a medical device — namely, of the “Instructions for Use” of Cidex OPA, which is used to high-level disinfection semi-critical devices, including gastrointestinal endoscopes.
For the reader’s convenience, this article is divided into 3 parts. The first and second parts are featured below.
The third part of this discussion, which provides guidance for the save and proper use of Cidex OPA, is provided in another of Dr. Muscarella’s article entitled “Recommendations for the Safe and Effective Use of ortho-phthalaldehyde“: click here to read it.
Although this article focuses on Cidex OPA, some of its content, including the provided recommendations, may also be applicable to other similar, products whose active ingredient is also OPA, which is known generically as ortho-phthalaldehyde.
A PRACTITIONER’s QUESTION
“I recently learned that under some circumstances Cidex OPA is contraindicated for processing urological instrumentation. But I am confused, because the label insert my endoscopy unit has on file for Cidex OPA does not include this contraindication. Dr. Muscarella, please explain the details of this contraindication and why my unit’s label insert does not include it.”
DR. MUSCARELLA’S RESPONSE
Cidex OPA is a liquid chemical sterilant/disinfectant (LCS) used to high level disinfect gastrointestinal (GI) endoscopes and other types of reusable semi-critical instruments.
This product, which is often referred to simply as “OPA” because of its active ingredient—0.55% ortho-phthalaldehyde—is a clear, light blue reusable solution that provides healthcare facilities with an alternative to 2% glutaraldehyde, such as Cidex.
Although its chemical structure classifies it as an aldehyde, Cidex OPA is different from, and is not to be confused with, Cidex or another glutaraldehyde solution (such as Metricide).
Cidex OPA has a slightly alkaline pH of 7.5; does not require chemical activation; is rapidly tuberculocidal; can be reused for as many as 14 days; has a shelf-life of up to 75 days; and, like most LCSs, requires that its concentration be monitored for effectiveness. Like Cidex, Cidex OPA is marketed and distributed by Advanced Sterilization Products (ASP).
Primarily because of its short immersion time, Cidex OPA may be favored by busy endoscopy units.
Whereas 2% glutaraldehyde solutions usually require 20 or 45 minutes to achieve high-level disinfection, Cidex OPA is labeled to achieve high-level disinfection in 12 minutes (at a minimum temperature of 20o C, or 68o F, which is room temperature), making it one of the first LCSs marketed in the U.S. to achieve high-level disinfection in less than 20 minutes.
Today, other disinfectants, such as Resert XL and Rapicide OPA-28, may achieve high-level disinfection in less than 10 minutes at room temperature. (Refer to the individual product’s labeling.)
Medical department that might use Cidex OPA to reprocess flexible endoscopes and other types of reusable semi-critical instruments include gastrointestinal endoscopy, bronchoscopy, urology, cardiology, gynecology, and the operating room.
Demonstrating a shift in a long-standing regulatory paradigm, the FDA cleared Cidex OPA in 1999 without a specific “sterilization” claim.
All previously cleared LCSs labeled for reprocessing flexible endoscopes include a label claim not only for high-level disinfection (typically during a short immersion time of, for example, 45 minutes), but also for “sterilization” (typically during a long immersion time of, for example, 10 hours).
Though the label of Cidex OPA does not include a “sterilization” claim, the company has confirmed that during sporicidal testing Cidex OPA satisfied the current regulatory requirements to claim “sterilization” during an exposure time and temperature of 32 hours and 20o C, respectively.
METHODS
As a result of this practitioner’s question and understandable confusion about contraindications associated with Cidex OPA, this product’s labeling was reviewed to determine whether Cidex OPA is contraindicated, under some circumstances, for processing urological instrumentation and, if so, why this contraindication is not included in the label that this inquiring practitioner’s endoscopy unit has on file.
Further, this article’s review is divided into three parts. The first provides this review’s results. The second discusses the significance and implications of these results. And the third part provides a number of recommendations for the safe and proper use of Cidex OPA (and other, similar high-level disinfectants whose active ingredient is ortho-phthalaldehyde, including Metricide OPA Plus and Rapicide OPA 28).
PART 1 – THE RESULTS
This review revealed that the labeling of Cidex OPA has been changed twice since 1999, resulting in the publication of (at least) three different versions of labeling, each version containing additional information regarding the safe use of Cidex OPA.
Salient information and instructions that are provided in the first and original label of Cidex OPA are highlighted in Table 1 (embedded below).
The two subsequent versions of Cidex OPA’s labeling, which are different from one another, are discussed in Table 2 and Table 3 (which are also embedded, further below) and contain significant changes and additions to Cidex OPA’s original labeling. (Due to space constraints, not all of the contents of each respective version of Cidex OPA’s label are listed in Tables 1-3.)
A side-by-side comparison of these three tables displays both the differences between each of these three versions of Cidex OPA’s label and the significant additions to each subsequent version. — Lawrence F Muscarella PhD
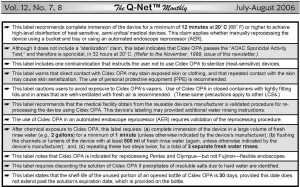
Table 1. Original Cidex OPA label (1999). Important information about the safe use of Cidex OPA that is included in its first and original label. Some of these instructions, such as the requirement to use Cidex OPA in a well-ventilated area to minimize exposure to its vapors, apply to virtually all types of liquid chemical disinfectant/sterilants (LCSs) used to reprocess endoscopes.
A. The original label (1999): The first and original label of Cidex OPA, published and cleared by the FDA in 1999, provides a singular contraindication that instructs the user not to use Cidex OPA to sterilize (heat-sensitive) medical instruments (see: Table 1).
This specific contraindication provides additional information, stating that high-level disinfection of rigid endoscopes is recommended by the Centers for Disease Control and Prevention (CDC), among other organizations, whenever the use of a biologically-monitored sterilization process is not feasible.
Moreover, in addition to providing the time and temperature required for Cidex OPA to achieve high-level disinfection — namely, 12 minutes at 20o C — Cidex OPA’s original label instructs users, as with all types of LCSs, to wear personal protective equipment (PPE) (e.g., gloves, gowns, and eye-wear) and to use Cidex OPA only in well-ventilated areas, to minimize exposure of personnel to its vapors (Table 1).
This label emphasizes that direct contact with Cidex OPA may stain exposed skin or clothing, and that rinsing the instrument three times with fresh water after immersion in Cidex OPA is essential to remove chemical residue and prevent patient injury.
Cidex OPA’s original label does not include a contraindication regarding its use to process urological instrumentation, such as cystoscopes. (For quality control and reference purposes, the original label of Cidex OPA may contain the designation “LC 20390-003 Rev. A” and the code “ASP 1999” at the end of its text.)
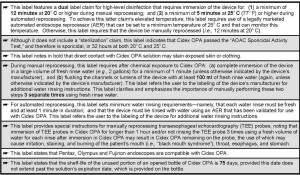
Table 2. Second Cidex OPA label (2003). Additional information about the safe use of Cidex OPA that is not included in the first and original version of its label (see: Table 1).
B. The second label (2003): The use of a product in the clinical setting can result in observations, applications, and important information not addressed in the product’s original label or 510(k) submission.
This review of Cidex OPA’s labeling and product literature revealed that ASP received clearance from the FDA in 2003 to modify Cidex OPA’s original label, the reason for which was primarily to provide users with updated information and a second claim to achieve high-level disinfection.
Whereas Cidex OPA’s original label provided only one claim of 12 minutes at 20o C (68o F) to achieve high-level disinfection (Table 1), this product’s revised (second) label provided an additional claim of 5 minutes at 25o C (77o F) to achieve high-level disinfection (Table 2; embedded above).
According to this second label, this elevated temperature claim requires use of a legally marketed automated endoscope reprocessor (AER) that can heat Cidex OPA to a minimum of 25o C. Immersion of an instrument in Cidex OPA for 5 minutes at a temperature below 25o C can pose an infection risk.
These and other salient changes to Cidex OPA’s original label are listed in Table 2.
Among other additions and changes, this revised (second) label uses capitalized letters to clarify and emphasize that during manual reprocessing the instrument must be rinsed with water after chemical immersion three separate times, using a fresh and large volume of water (e.g., 2 gallons) for each rinse.
Sidebar: Under its heading “Sterile water rinse,” Cidex OPA’s label states that: “Devices intended for use in known immuno-compromised patients, or potentially immuno-compromised patients based on institutional procedures (e.g., high-risk population served). Dr. Muscarella would suggest that this labeling claim might be inconsistent with “Standard Precautions,” which requires that certain measures be universally in place, regardless of information about or knowledge of the patient’s infection and/or immune status.
As significant, Cidex OPA’s revised label provides special instructions for reprocessing trans-esophageal echocardiography (TEE) probes that were not provided in Cidex OPA’s original label, including:
- not to immerse TEE probes in Cidex OPA for more than an hour (or for less than 12 minutes at room temperature, or 68o F), because excessive soaking (and/or inadequate water rinsing can cause the device to retain residual disinfectant, posing an increased risk of irritation or chemical burns to the patient’s mouth, throat, esophagus and stomach during the procedure; and
- to ensure that TEE probes are rinsed with water in accordance with Cidex OPA’s instructions (i.e., three separate fresh water rinses).
Like its original label, Cidex OPA’s revised (second) label does not include a contraindication regarding its use to process urological instrumentation, such as cystoscopes. (This revised label provides only one contraindication–the same one provided in Cidex OPA’s original label–that instructs the user not to use Cidex OPA to sterilize heat-sensitive medical devices.)
C. Product notification—The third label (2004, 2006): ASP wrote a “product notification” letter, dated April, 23, 2004, that notified customers of another significant modification to Cidex OPA’s label.
This letter stated that in rare instances Cidex OPA has been associated with anaphylaxis-like reactions experienced by patients with bladder cancer undergoing repeated cystoscopies (see: Table 3, which can be read by downloading the complete PDF version of this article “Cidex OPA: A Tale of 3 Labels” – click here to download it).
Additionally, this letter reported that in rare instances healthcare workers experienced an irritation or a possible allergic reaction that may have been associated with exposure to Cidex OPA; for its part, ASP noted in this letter that in most of these cases healthcare workers were not using Cidex OPA in accordance with its “Instructions for Use” (IFU). (ASP notified its customers and healthcare professionals a second time of these reports by way of another letter dated January 3, 2005.)

Table 3. Third Cidex OPA label (2004, 2006). Click here to download the complete PDF version of this article “Cidex OPA: A Tale of 3 Labels,” which includes Table 3. Additional information about the safe use of Cidex OPA that is not included in either the first and original version of its label (see: Table 1, above) or the second version of its label (see: Table 2, above).
These findings and clinical reports discussed in ASP’s “production notification” letter were the basis for ASP to update and modify Cidex OPA’s label for a second time.
This modified (third) label was published in 2004 and, as displayed in Table 3, for the first time includes the contraindication that Cidex OPA is not to be used to process any urological instrumentation used to treat patients with a history of bladder cancer.
This third label was published in 2004 and, as displayed in Table 3, for the first time includes the contraindication that Cidex OPA is not to be used to process any urological instrumentation used to treat patients with a history of bladder cancer. — Lawrence F Muscarella PhD
Click here to download the complete PDF version of this article “Cidex OPA: A Tale of 3 Labels,” which includes Table 3.
PART 2 – SIGNIFICANCE AND IMPLICATIONS
There are a number of significant implications associated with the instructions provided in Cidex OPA’s labeing, which has been modified twice since 1999—the year Cidex OPA was cleared by the Food and Drug Administration (FDA) for marketing.
A side-by-side comparison of Tables 1 – 3 (above) facilitates a clearer understanding of the contents of, additions to, and salient differences between, each of the three versions of Cidex OPA’s label.
Cidex OPA is contraindicated for reprocessing urological instrumentation to be used to treat patients with a history of bladder cancer. — Lawrence F Muscarella PhD
The three different versions of Cidex OPA’s label—published sequentially between 1999 and 2006—are a result, in part, of new clinical data that became available subsequent to Cidex OPA’s introduction onto the market.
It is unclear whether most reprocessing staff members are aware that three different versions of Cidex OPA’s label have been published, and that only in its most recent, or third, version does an important contraindication appear. (The third version of Cidex OPA’s label can be distinguished from its previous two versions by the unique text “ASP, 2004,” “ASP, 2006,” or “mailer, 4/04” appearing at the end of the label.)
Failure to review, understand, and comply with the instructions, precautions, and contraindications detailed in the most recent version of Cidex OPA’s label can increase the risk of ineffective reprocessing and injury to both patients and healthcare staff members.
This article provides some rare insight into the evolution of the labeling of a popular liquid chemical sterilant/disinfectant (LCS) used to reprocess different types of reusable. — Lawrence F Muscarella PhD
— Cidex OPA is contraindicated for reprocessing any urological instrumentation used during a procedure, such as cystoscopy, to treat patients with a history of bladder cancer: Whereas 2% glutaraldehyde is indicated for reprocessing virtually all types of flexible endoscopes, Cidex OPA (and most other high-level disinfectants containing ortho-phthalaldehyde) is contraindicated for reprocessing urological instrumentation, such as cystoscopes, to be used to treat patients with a history of bladder cancer.
This specific contraindication is included in the third version of Cidex OPA’s label. (Cidex OPA is not contraindicated for reprocessing GI endoscopes and bronchoscopes.)
Note that the labeling of one ortho-phthalaldehyde product – CIDEX OPA Concentrate ortho-phthalaldehyde High Level Disinfectant Concentrate (which is validated for use only in the EvoTech Endoscope Cleaner and Reprocessor System) – does not explicitly contraindicate this high-level disinfectant for reprocessing urological instrumentation.
This review’s identification of three different versions of Cidex OPA’s label — only the third of which includes an important contraindication regarding urological instrumentation — would explain the nurse’s question about Cidex OPA (see the top of this article).
As this nurse’s question would suggest, the version of Cidex OPA’s label that this nurse’s endoscopy department was using and had on file was likely either its first or second, both of which were published prior to 2004–the year that its manufacturer mailed a “product notification” letter to customers, along with a copy of the third (and current) version of Cidex OPA’s label, highlighting the addition of this contraindication for reprocessing urological instrumentation to be used to treat patients with a history of bladder cancer.
— The label of Cidex OPA does not include a “sterilization” claim: Prior to the FDA’s clearance of Cidex OPA in 1999, virtually all LCSs marketed in the U.S. for reprocessing flexible endoscopes and other types of semi-critical reusable devices were labeled to achieve high-level disinfection and “sterilization” during relatively short and long exposure times, respectively.
Despite it having demonstrated sporicidal properties (click here and review this link’s Table 5 on page 3), Cidex OPA was the first to be cleared by the FDA as a high-level disinfectant without its labeling including a “sterilization” claim.
The labels of virtually all 2% glutaraldehyde solutions cleared by the FDA more than a decade ago, for example, claim to achieve high-level disinfection in 45 minutes and “sterilization” in 8 or 10 hours (at 25o C). Somewhat contrary to regulatory tradition, Cidex OPA was cleared by the FDA as a high-level disinfectant without a “sterilization” claim.
The medical literature indicates, however, that use of a high-level disinfectant whose label does not include a “sterilization” claim, such as Cidex OPA (and other similar high-level disinfectants containing ortho-phthalaldehyde and, too, the Sterilox high-level disinfectant), is not clinically problematic and does not increase the risk of healthcare-associated infection.
Its label’s lack of a “sterilization” claim notwithstanding, Cidex OPA is reported to destroy high numbers of bacterial endospores during the AOAC Sporicidal Test, albeit in 32 hours at 20o C and 25o C. This standardized sporicidal test is an important benchmark that must be satisfied to label a LCS to achieve “sterilization.”
Whether the FDA’s clearance of Cidex OPA as a high-level disinfectant without a “sterilization” claim reflects a shift in a long-standing regulatory paradigm that is in response to several published articles that question the appropriateness and scientific validity of labeling a LCS to achieve “sterilization” is unclear.
Labeling as “100% sporicidal” (at a specified immersion time and temperature) a high-level disinfectant or sterilant that has passed, among other tests, the AOAC Sporicidal Test may be more appropriate and scientifically valid than labeling the product to achieve sterilization. — Lawrence F Muscarella PhD
Because high-level disinfection prevents disease transmission, it is not surprising that endoscopes that have been properly cleaned, high-level disinfected, rinsed with clean water, and dried with forced air have not been associated with disease transmission.
While there is an academic distinction between high-level disinfection and sterilization, clinical differences between the two have not been demonstrated in the endoscopic setting.
High-level disinfection destroys all types of pathogens encountered in the clinical setting, including the hepatitis C virus, HIV, Mycobacterium tuberculosis, which is the
causative agent of pulmonary tuberculosis, and Clostridium difficile–a spore-forming bacterium that may be encountered during lower GI endoscopy. — Lawrence F Muscarella PhD
— No liquid chemical sterilant is ideal and without shortcomings: Some of the warnings and precautions included on its label are not necessarily unique to Cidex OPA and may also be included on the labels of other LCSs used to reprocess reusable devices. No LCS is ideal and without potentially significant shortcomings.
For example, reports of medical staff members experiencing respiratory sensitization following repeated exposure to 2% glutaraldehyde have been documented. Moreover, the MSDS (“material-safety-data-sheet”) of 0.2% peracetic acid and 7.5% hydrogen peroxide—two other LCSs used to reprocess flexible endoscopes—state that, among other safety concerns, both can cause irreversible eye damage and be corrosive to skin and mucous membranes (and delicate instruments).
Indeed, Cidex OPA is not the only high-level disinfectant that requires, for example, that reprocessing staff members wear personal protective equipment (PPE) and that the reprocessing room be ventilated with at least 10 room exchanges of fresh air per hour.
In truth, Cidex OPA has some advantages compared to several other LCSs, such as its claim to achieve high-level disinfection in 5 minutes at a minimum of 25o C.
But, despite this and other advantages, Cidex OPA (and other comparable products) is contraindicated for reprocessing urological instrumentation to be used to treat patients with a history of bladder cancer (see above).
— Cidex OPA’s high-level disinfection claim at an elevated temperature contraindicates manual reprocessing: In 2003, the manufacturer of Cidex OPA received clearance by the FDA to modify Cidex OPA’s original label, cleared in 1999 with the claim to achieve high-level disinfection in 12 minutes at 20o C, and to market Cidex OPA with the additional claim to achieve high-level disinfection in 5 minutes at 25o C.
Prior to the clearance of this second version of Cidex OPA’s label (in 2003), virtually all LCSs cleared by the FDA to achieve high-level disinfection at an elevated temperature are indicated for both manual and automated reprocessing.
For example, solutions of 2% (alkaline) glutaraldehyde, cleared by the FDA more than a decade ago and labeled to achieve high-level disinfection at 25o C, are indicated for both manual and automated reprocessing.
The labels of some recently cleared LCSs that claim to achieve high-level disinfection at an elevated temperature, even if only 5o C above room temperature (i.e., 25o C), however, contraindicate manual reprocessing and require their exclusive use in an automated endoscope reprocessor (AER).
If your AER cannot be set to a minimum of 25o C please follow the time and temperature stated in Indications for Use, Manual Processing. — Cidex OPA’s label
Cidex OPA, for example, must be used in a legally-marketed AER whenever Cidex OPA is used to achieve high-level disinfection in 5 minutes at an elevated temperature of 25o C. (Cidex OPA’s label does not contraindicate manual reprocessing, however, when used at room temperature during an immersion time of 12 minutes.)
The FDA typically requires that a legally-marketed automated endoscope reprocessor (AER) be used whenever a high-level disinfectant, including Cidex OPA, is used to achieve high-level disinfection at an elevated temperature (e.g., 25o C or 35o C).
Moreover, the label of Rapicide—a 2.5% (acidic) glutaraldehyde formulation that achieves high-level disinfection in 5 minutes at 35o C (i.e., 95o F)—also contraindicates manual reprocessing and requires the use of an AER.
Whether these clearances reflect a potential shift in another long-standing regulatory paradigm that would appear to suggest that the FDA no longer considers manual heating of LCSs safe or effective is unclear.
Further, Cidex OPA’s label (and the labeling of comparable products) requires that the AER be equipped with an immersion heater and be designed to automatically control and monitor Cidex OPA’s elevated temperature during high-level disinfection.
If the AER does not satisfy these requirements, then the reusable device must be manually reprocessed using Cidex OPA for 12 minutes at room temperature.
A drop in the temperature of Cidex OPA (or a comparable product) below 25o C during a 5-minute immersion time (or below 20o C during a 12-minute immersion time) poses an increased risk of infection and can result in disease transmission. — Lawrence F Muscarella PhD
Indeed, using, for example, an aquarium heater to heat a LCS manually, as well as manually controlling and monitoring the LCS’s elevated temperature during chemical immersion, can be problematic, cumbersome, and result in ineffective reprocessing.
Last, although elevating the temperature of a LCS typically increases its biocidal effectiveness, it may also increase the LCS’s vapor pressure, which, particularly during manual reprocessing, increases the potential for irritation and sensitization to the skin, noses, throats, and respiratory tracts of medical staff members.
Most AERs are designed with a tightly-fitting lid to minimize exposure of medical staff members and the surrounding environment to the LCS’s potentially irritating vapors, which might further explain why the FDA requires at least some LCSs associated with an elevated temperature claim to be used exclusively in an AER.
— The importance of water rinsing: In addition to minimizing exposure of medical staff members and the surrounding environment to an LCS’s potentially irritating vapors, there is another reason why the FDA might favor, if not encourage, the routine use of an AER, especially when using Cidex OPA.
While manual reprocessing as a discipline has not been demonstrated to be associated with a higher incidence of healthcare-associated infection than automated reprocessing, the former is inherently prone to variability and an inconsistent outcome.
AERs, however, standardize several reprocessing steps, including water rinsing, ordinarily allaying concerns that a crucial reprocessing step was skipped or overlooked.
Automated endoscope reprocessors (AERs) standardize several reprocessing steps, including water rinsing.
Patient injury due to failure of water rinsing to remove all of the chemical residue from the surfaces of a reusable device, such as a TEE (or, “transesophageal echocardiography”) probe, has been reported (refer to the next section, below). LCSs that are not easily removed during rinsing due to their limited solubility in water would be of a particular concern.
Whereas the MSDS of Cidex (2% glutaraldehyde) confirms that it is “completely soluble” in water, the MSDS of Cidex OPA lists it simply as “soluble” in water, which suggests that Cidex OPA is less soluble in water than Cidex and, therefore, harder to remove from a reusable device during water rinsing.
The importance of effective water rinsing is underscored by all three versions of Cidex OPA’s label, which specify that during manual reprocessing the reusable device must be rinsed three separate times with fresh water following chemical immersion, to prevent potentially harmful residue of Cidex OPA from remaining on the device.
Attention — The “3-2-1” recommendation: Instruments reprocessed using Cidex OPA (or a comparable product require 3 separate water rinses, each rinse of 2 gallons of fresh, clean tap (or sterile) water for a minimum of 1 minute in duration.
Each labeling version further specifies that, for each of these three rinses, the reusable device must be completely immersed in a large volume of fresh water (“2 gallons”) for a “minimum of 1 minute in duration.” (The second and third versions of Cidex OPA’s label recommend that the lumens or channels of a reusable device be flushed with not “less than 100 mL [or milliliters] of rinse water” during each separate rinse.)
While a potential concern to patient safety, any deviation from these specific water rinsing parameters and instructions — for example, rinsing the reusable device only once with water after high-level disinfection — might be permissible, but arguably only if, among other considerations, the manufacturer of the reusable device during manual reprocessing, or the manufacturer of the AER during automated reprocessing, provides the user with validated and “FDA-approved” data clearly demonstrating that the alternative water rinsing procedure completely removes potentially harmful residue of Cidex OPA (or another LCS) from all of the device’s surfaces.
None of the three versions of Cidex OPA’s label, however, specifies the number of water rinses, or the minimum water volume for each rinse, that are required during automated reprocessing, even though both parameters are important to rinsing a reusable device successfully after chemical immersion using an AER.
Instead, Cidex OPA’s label assigns the responsibility of determining these water rinsing parameters to the manufacturer of the AER (see below), stating:
The use of Cidex OPA in automated endoscope reprocessors must be part of a validated reprocessing procedure. The contact conditions must be 25o C for 5 minutes. — Lawrence F Muscarella PhD
— Reprocessing TEE probes using Cidex OPA: TEE probes are delicate instruments used non-invasively to provide clear ultrasound images of the functioning heart. Resembling a flexible GI endoscope without any internal channels, TEE probes are introduced during TEE into the upper gastrointestinal (GI) tract via the patient’s oral cavity.
The 2nd and 3rd versions of Cidex OPA’s label provide special instructions for (manually) reprocessing these probes. Both versions caution that ineffective water rinsing due to immersion of the TEE probe in Cidex OPA for longer than an hour, and/or not rinsing the TEE probe three separate times with fresh water after chemical immersion, may result in chemical burns, irritation, and staining of the patient’s mouth, throat, esophagus, and stomach.
Because the first version of Cidex OPA’s label does not include these specific instructions for reprocessing TEE probes, the inclusion of these instructions in the second and third versions of Cidex OPA’s label would reasonably suggest that sometime between 1999 and 2003 — the year the second version of Cidex OPA’s label was published — Cidex OPA’s manufacturer received reports associating the potential for patient injury to inadequately rinsed TEE probes reprocessed using Cidex OPA.
— Whose instructions are to be followed? In addition to providing important instructions, precautions, and contraindications, all three versions of its label assign some of the responsibility of using Cidex OPA to the manufacturer of the reusable device and, as discussed in a previous section, to the manufacturer of the AER, if one is used.
In addition to none of the three versions of Cidex OPA’s label providing the number of water rinses or the minimum water volume per rinse that is required to rinse any type of reusable device successfully with water during automated reprocessing, all three versions recommend:
1. “Refer to the reusable medical device manufacturer’s labeling for additional (water) rinsing instructions”;
2. “The reusable device manufacturer should provide the user with a validated reprocessing procedure for that device using Cidex OPA Solution”;
3. “The use of Cidex OPA with semi-critical devices must be part of a validated (water) rinsing procedure as provided by the (reusable) device manufacturer”;
4. Immerse the reusable device during each rinse “for a minimum of 1 minute in duration” unless the device’s manufacturer specifies a longer time; and
5. The use of Cidex OPA Solution in automated endoscope reprocessors (AERs) “must be part of a reprocessing (and water rinsing) procedure (provided and validated by the manufacturer of the AER).”
Additionally, the second and third versions of Cidex OPA’s label provide a 6th recommendation:
6. “Select a rinse cycle on an automatic endoscope reprocessor that has been validated for use with” Cidex OPA.
While most of the reprocessing instructions detailed in all three versions of Cidex OPA’s label are clear and sufficient for effective reprocessing and water rinsing, these six recommendations demonstrate that some of the instructions provided by the three versions of the label of Cidex OPA (and other LCSs) are limited and require cooperation by, and both information and input from, the manufacturer of the reusable device and the manufacturer of the AER, if one is used.
Whereas the first four of these six recommendations instruct healthcare staff to obtain reprocessing instructions and validated data from the manufacturer of the reusable device, the 5th and 6th recommendations instruct the user to contact and obtain validated data from the manufacturer of the AER.
— Which raises the following questions: What if the label (and manufacturer) of the reusable device and/or the label of the AER provides a reprocessing recommendation, instruction, or contraindication that is inconsistent with the label and reprocessing instructions of the LCS? Which label and reprocessing instructions should the user follow?
Additional questions arise: l What is a medical facility to do if, in an attempt to comply with, for example, the 2nd and 5th recommendations (above), it requests from the manufacturers of both the reusable device and the AER a copy of these validated procedures and data, but neither manufacturer complies, stating that these data are “proprietary” and not available for public review?
And, what if a patient is injured as a result of residue of Cidex OPA remaining on a reusable device that was inadequately rinsed with water by an AER? Which of these three manufacturers would be accountable and to blame? Medical facilities seek clear advice to these and other important reprocessing questions.
PART 3 – RECOMMENDATIONS FOR THE SAFE AND PROPER USE OF CIDEX OPA
When used in accordance with its label, Cidex OPA (and comparable products whose active ingredient is ortho-phthalaldehyde), which is not to be confused with Cidex (2% glutaraldehyde), is reported to be safe, effective and a valued addition to an endoscopy department’s armamentarium of instrument reprocessing products.
Recommendations
Recommendations for the safe and proper use of Cidex OPA are provided in this article’s third part —– click here to read it — which, also authored by Dr. Muscarella, is entitled “The Safe and Effective Use of ortho-phthalaldehyde.”
These recommendations are an adjunct to, not a replacement for, the most recent (third) version of Cidex OPA’s label. Many of these recommendations are not unique to Cidex OPA and are also provided for the safe and proper use of other LCSs.
Article by: Lawrence F Muscarella, PhD; posted: 5-6-2013; revised 12/23/2014, Rev A.
A very good article that answered a number of my questions, thank you.