January 5, 2015 — This article provides a number of recommendations to prevent disease transmission and manage risk during flexible endoscopy. Diagrams of GI endoscopes are also provided as a tool to explain the design of the GI endoscope and the function of both the colonoscope’s auxiliary water channel and its air/water channel system.
Background
Spotlighting the importance of proper instrument reprocessing as much to the prevention of disease transmission as to a medical facility’s quality and management of risk, a jury in a western Pennsylvania county, in July, 2012, found a hospital “negligent” for having improperly cleaned and high-level disinfected GI endoscopes.(1)
Six years earlier, in February, 2005, this hospital—located near Pittsburgh (PA) and referred to herein as Hospital A—learned that for almost 4 months it had not been reprocessing an internal channel featured in one of its models of GI endoscopes.
Two of this hospital’s colonoscopes were of this model type, and, posing an increased risk of disease transmission, both were used on a total of more than 200 patients between October, 2004, and February, 2005.(1-4)
Within weeks, in March, 2005, Hospital A mailed certified letters to these affected patients notifying them of this colonoscope-reprocessing breach and its potential to have exposed each patient to infectious agents, including bacteria, the hepatitis B and C viruses and HIV.(1-4)
Click here to read Dr. Muscarella’s related article entitled, “How to reprocess the MAJ-855 water tube and the GI endoscope’s auxiliary water channel.”
A study of this infection-control lapse not only reveals some interesting legal facts, but also provides a timely opportunity to review, if not to learn for the first time, a number of reprocessing principles and instrument-design features that are crucial to the prevention of healthcare-associated infections.
Legal Reviews for Consumers, Hospitals, Manufacturers: Click here to read about Dr. Muscarella’s expertise and legal assessments of the causes of healthcare-associated infections, including “superbug” outbreaks linked to contaminated GI endoscopes and other reusable medical equipment.
Details of the lawsuit
Due to the potential for this reprocessing breach to have resulted in patient-to-patient disease transmission, two of these more than 200 affected patients sued Hospital A on April 14, 2005, just days after both had received Hospital A’s certified letter acknowledging this medical error.(2-4)
In their lawsuit these plaintiffs requested (by way of legal counsel) that the court not only grant class action status to their complaint, but also award the claimants compensatory and punitive damages.
The class-action lawsuit that provides important details about Hospital A’s breach may be read by clicking here.(3)
This jury’s verdict permits individual trials to proceed and determine whether these plaintiffs might be entitled to damages for, but not limited to, “mental anguish,” “embarrassment and humiliation,” and “pain and suffering.”(3)
Details of the breach
The timeline and details of Hospital A’s colonoscope-reprocessing breach are provided in Table 1 (“A Timeline of Events,” embedded below).
Briefly, according to the plaintiffs’ filed lawsuit and other published reports,(1-4) Hospital A began using two new colonoscopes, both of the model: CF-Q160AL (manufacturer: Olympus America),on October 28, 2004.
Almost four months later, in early February, 2005, staff observed fecal matter dripping from one of these two colonoscopes after the instrument had been ostensibly “reprocessed.”(1) Staff suspected that damage to the GI endoscope might be the cause of this dripping debris.
Note: Although GI endoscopes manufactured by Olympus America are the subject of this article, the details and recommendations provided herein are not necessarily unique to this one manufacturer and may also apply to similar types of GI endoscopes (and other flexible endoscopes) marketed and sold by other manufacturers.
Hospital A, therefore, had the colonoscope serviced; determined, however, that it was not damaged; and subsequently reused the colonoscope on patients.(1)
A few weeks later, fecal matter was similarly observed to be dripping from the other of these two recently-purchased colonoscopes (same model: CF-Q160AL).(1)
The hospital had this colonoscope serviced, too, only to learn that, as with the other colonoscope, it was not damaged and was functioning as intended.
Shortly thereafter, by the end of February, 2005, Hospital A had stopped using both of these two suspect colonoscopes and contacted their manufacturer (Olympus America) to discuss the possible causes of this apparent reprocessing breach.(1)
During this discussion Hospital A learned that both of these colonoscopes (which the hospital had been using on patients since October, 2004) were of a model type that featured an auxiliary water channel.
Although the hospital had not been doing so, this channel requires cleaning and high-level disinfection after each procedure to prevent disease transmission,(1) whether or not the channel was used during the examination.
As if a case of deja vu, a newspaper reports in July, 2013, that a hospital in the country’s heartland notified patients of their possible exposure to HIV and the hepatitis B and C viruses, due to the inadvertent failure to clean and disinfect the colonoscope’s auxiliary water channel.

Due to this finding, Hospital A soon notified the more than 200 affected patients (on whom either of these two colonoscopes was used) of this infection-control breach (see: Table 1, embedded to the right.(1-4)
(Note: A distinction without a difference, whereas one manufacturer may refer to this dedicated channel as the “auxiliary water channel,” another manufacturer may refer to it as a “forward water jet channel.”)
Click here read Dr. Muscarella’s related article entitled, “Improper use and reprocessing of a GI endoscope’s auxiliary water system.“
The breach’s root cause
The root cause of these two recently purchased colonoscopes remaining soiled with fecal matter, despite having been “reprocessed,” reportedly was the unwitting failure to have reprocessed the dedicated auxiliary water channel—whose function and intended use are described in Box A (also see: Figure 1, embedded below).
A number of factors contributed to Hospital A’s colonoscope-reprocessing breach. For example, the hospital reportedly lacked training and knowledge about the internal designs of these two recently-purchased colonoscopes (model: CF-Q160AL), and it was unaware that both featured a dedicated auxiliary water channel requiring reprocessing.(1-4)
A related contributing factor, Hospital A’s other, older colonoscope models in inventory did not feature this specialized channel,(1,3) an incongruity that understandably might have caused diligent staff members confusion.
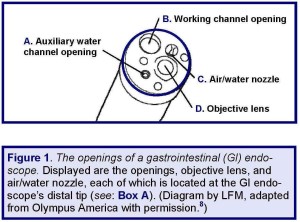
Moreover, colonoscopes equipped with an auxiliary water channel are not necessarily easily distinguishable from those that are not. It is true that GI endoscopes with an auxiliary water channel feature a distinct auxiliary water inlet (that provides access to this channel for both its clinical use and reprocessing). But, the location of this inlet may not be obvious to untrained staff members.
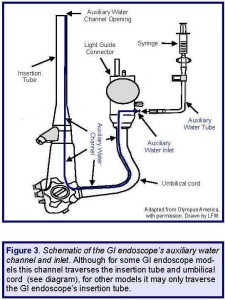
For some models of GI endoscopes, such as for Hospital A’s two suspect colonoscopes, this inlet is located on the light guide connector (see: Figures 2 and 3), whereas for other models, this inlet may be located on another section of the GI endoscope—for example, on its control section (e.g., model: CF-1T140L; see: Box A).(5)
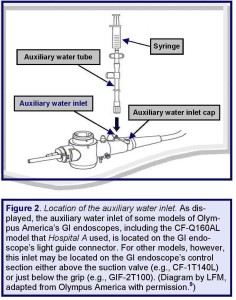
Yet another related factor that might have contributed to Hospital A’s infection-control lapse, a GI endoscope with an auxiliary water inlet is not necessarily also equipped, de facto, with an auxiliary water channel.
Some GI endoscopes without this specialized channel nevertheless feature an auxiliary water inlet that may be located just below the grip (e.g., the CF-130 series of videoscopes).
For these models of GI endoscopes, the inlet is a “side-tap” that provides access to the standard water channel.(5)
The degree of concern that a specific instrument-reprocessing breach may cause is a consequence of the breach’s assessed risk of infection. One fact not disputed in Hospital A’s legal case is that the failure to have reprocessed the GI endoscope’s auxiliary water channel posed an increased risk of infection.(6)
A salient omission?
Nevertheless, while the aforementioned lawsuit asserts that the plaintiffs have “suffered injury,”(3) notably missing in this lawsuit is the plaintiffs’ claim that any were infected as a consequence of Hospital A’s reprocessing breach.
Indeed, a recent news report published this past summer stated that: “none of the patients (affected by Hospital A’s breach) contracted a disease as a result of their exposure”(1)—the jury’s verdict of negligence notwithstanding.
This hospital’s breach, plaintiffs’ legal case, and jury’s verdict present a surprising outcome with significant implications:
that a medical facility can be sued and found legally responsible (i.e., negligent), even if the instrument-reprocessing breach that is the focus of the lawsuit had not been documented to cause an infection.
(Note: The lack of infection does not ensure that a breach was not associated with disease transmission. Depending on the circumstances [e.g., the patient’s health], an infectious disease can be transmitted to a patient without the patient eliciting symptoms of infection. Some infections may also be asymptomatic or sub-clinical).
Inauspicious circumstances
The use of a GI endoscope that features an auxiliary water channel would not ordinarily have been a source of confusion, were it not possibly for the combination of a number of inauspicious circumstances.
First, Hospital A’s older, familiar models of colonoscopes in its inventory were not equipped with an auxiliary water channel. Indeed, the apparent misconception that, therefore, the two new models of colonoscopes (sold by the same manufacturer) that Hospital A purchased would similarly not feature this specialized channel was reportedly an important factor that contributed to this hospital’s error.(1-4)
A second complicating consideration, to an untrained staff member colonoscope models in inventory with, and without, an auxiliary water channel may not be easily identifiable, which can cause confusion.
Moreover, mistaking one model of a colonoscope for another, or erroneously concluding that the reprocessing requirements of old and new colonoscope models sold by the same manufacturer do not markedly differ, in addition to posing an increased risk of infection, brings into focus a third factor that, in part, reportedly contributed to Hospital A’s error:
namely, the employment of a quality-assurance program that was not sufficiently robust to ensure that every one of the internal channels—namely, the auxiliary water channel—of each old and new models of GI endoscopes in Hospital A’s inventory was being reprocessed.
Recommendations
This study of Hospital A’s reprocessing error yields several recommendations that may be useful to a medical facility aiming to enhance patient safety, improve its quality-assurance program and endoscope-reprocessing practices, and minimize its potential legal exposure.
1. Employ a robust quality-assurance program that, among other responsibilities, ensures reprocessing staff members are trained and understand the internal design and reprocessing requirements of every GI endoscope model in inventory.
– Perform routine audits of staff members’ reprocessing practices, correcting any noted deficiencies.
› Such audits and training may be especially important if the medical facility uses different models and types of GI endoscopes; and/or has recently added a new model or series of GI endoscopes to its inventory. Remember that the internal designs and reprocessing requirements of new models of GI endoscopes may be unique, unlike older models, and require additional reprocessing steps.
2. In particular, ensure that every one of the (accessible) internal channels of every GI endoscope in inventory is being properly reprocessed after each procedure, whether or not the channel was used during the clinical examination.
– Identify those GI endoscopes in inventory featuring an auxiliary water channel (or another specialized channel) and confirm the location of the auxiliary water inlet. Ensure that the auxiliary water channel is being reprocessed using the proper adapter (e.g., MAJ-855 tube).
› Caution is advised whenever models of GI endoscopes that do, and do not, feature an auxiliary water channel (or other specialty channel) are being used. These models are not necessarily easily distinguishable.
– Also, ensure that the GI endoscope’s valves and other potentially contaminated internal and external surfaces are being properly reprocessed.
3. Additional recommendations are provided on request.
Article by: Lawrence F Muscarella, PhD, posted 10-15-2012; updated 1-5-2015 Rev A.
I came accross this website to read more info on this great product: http://www.medicalexpo.com/medical-manufacturer/endoscope-522.html