August 15, 2013 (updated: January 27, 2015) — This article by Dr. Muscarella is the 2nd in a series of three that discusses disposable tubing used for irrigation during gastrointestinal (GI) endoscopy.
A more complete version of this article’s product review, written in PDF format and containing all of the several tables (Tables 1-5), figures (Figures 1-4) and box articles (Boxes A-C) discussed below, may be read by clicking here.
BACKGROUND
Specifically, this 2nd article focuses on the features, designs, and labeling of disposable irrigation tubing used during gastrointestinal (GI) endoscopy.
The other two articles, which complete this series, focus on the reusable components of the auxiliary water system and on guidance for the safe use of disposable irrigation tubing, respectively. These two are entitled:
- “Improper Use and Reprocessing of a Gastrointestinal Endoscope’s Auxiliary Water System” — click here to read the 1st article in this series. The more complete version of this first article in this series, in PDF format, may be read by clicking here; and
- “Guidance for the Safe Use of “Disposable” Irrigation Tubing Used During GI Endoscopy” — click here to read the 3rd article in this series. The more complete version of this 3rd article in this series, in PDF format, may be read by clicking here.
This series of three is this the most complete set of articles focusing on this topic.
INTRODUCTION
The first article in this series discusses the potential for disease transmission associated with several infection-control breaches confirmed at the two Veterans Affairs Medical Centers (VAMCs) in Murfreesboro (TN) and Miami (FL), in 2008 and 2009, respectively.
Table 1 summarizes the focal points of the first article in this series.[1] It is only available in this article’s more complete PDF version — click here for a copy.
As a result of these infection-control breaches, along with those contemporaneously identified at the VAMC in Augusta (GA), the Veterans Health Administration (VHA), in 2009, informed almost 10,000 affected patients of their potential exposure to blood-borne viral diseases during flexible endoscopy.[2]
These breaches received national attention in 2009 and, as the reader may recall, were not only the primary focus of an investigative report published by the Veterans Affairs Office of Inspector General (VA-OIG) (click here to read this report),[2] but were also the subject of a congressional hearing (click here) in Washington, DC.[3]
Reusable auxiliary water system
The several infection-control breaches identified by the VHA at these two VAMCs are listed in Box C of the first article in this series.[1]
One of these breaches—the improper reprocessing of a reusable component of an auxiliary water system (manufacturer: Olympus America),[2] namely, the MAJ-855 auxiliary water tube, (or, “AWT”; pictured here)—was identified at both of these VAMCs.
Whether reusable or disposable, irrigation tubing is routinely used to rinse the GI tract, which may be required to enhance visualization of the mucosa. — Lawrence F Muscarella PhD
Like comparable systems sold by other manufacturers for the same intended use, this reusable auxiliary water system provides for the irrigation of the gastric and colonic mucosa exclusively via the gastrointestinal (GI) endoscope’s auxiliary water channel (which is pictured here).
On occasion, such irrigation may be necessary to remove patient materials and debris, enhance visualization, and provide for the proper diagnosis and treatment of disease.

In addition to the GI endoscope’s auxiliary water channel itself, this reusable auxiliary water system features:
- the reusable AWT and other tubing;
- connectors;
- a water pump; and
- a water bottle (it may also feature a particulate water filter).[1]
(A summary of this article is embedded in the box to the right.)
Another of this reusable auxiliary water system’s tubing is the short OFP irrigation tube (or, “SIT”[1]; pictured here), which, along with the water bottle, this article defines as “reposable” (i.e. a single-day item).AA
The proper setup and use of this reusable system are discussed in the first article in this series (refer to that article’s Box A and Figure 1).[1]
Reader: Please refer to the end of this article for its “key,” which provides the footnote associated with the each of this blog’s supra-scripted double-capitalized letters, such as “AA.”
Despite the AWT (which is approximately 4-foot in length) being designed for use with a one-way valve whose primary function is to prevent this tubing’s contamination due to the “backflow” of potentially infectious patient materials and fluids during GI endoscopy, its manufacturer nonetheless labels the AWT to be reprocessed — that is, cleaned and either high-level disinfected or sterilized — after each GI endoscopic exam, apparently to further mitigate the potential risk of this tubing’s becoming a source of cross-infection.[1,2]
Infection control breaches
The improper reprocessing of the reusable (MAJ-855) AWT by both the Murfreesboro VAMC and Miami VAMC was a salient lapse (see: Table 1, which is only available in the more complete PDF version of this article — click here to download it).[1]
But the associated infection-control breach that received the most attention and discussion was the former VAMC’s inadvertent use of the AWT fitted with — not the correct one-way valve (with which its manufacturer designed the AWT to be used) — but a similarly colored and shaped, although improper, two-way connector (also manufactured by Olympus America) that is intended to be used with another reprocessing accessory: namely, the MH-974 washing tube (which is approximately 1-foot in length; pictured here).[1]
Displayed in the first article in this series,[1] this mix-up by the Murfreesboro VAMC facilitated the contamination of the AWT, due to the confirmed backflow of blood from a patient’s GI tract. (This backflow would have reasonably been prevented if the AWT had been fitted with the correct one-way valve.[2])
As a consequence of the AWT’s contamination — coupled with this VAMC’s reprocessing of this tube only once at the end of the day, not after each GI procedure as its manufacturer requires — the VHA notified 6,387 affected patients of the potential for an increased risk of infection with blood-borne pathogens during colonoscopy.[1-3]
Disposable irrigation tubing
The improper reprocessing of the auxiliary water system’s reusable AWT by the two VAMCs in Murfreesboro (TN) and Miami (FL) necessarily introduces for discussion whether disposable tubing might be a safe alternative for irrigation during GI endoscopy (see: Table 1, which is only available in the more complete PDF-version of this article — click here to download a copy).[2]
Different reprocessing instructions for components of the auxiliary water subsystem creates confusion. — The VA-OIG.[1,2]
Two brands of “disposable”BB irrigation tubing with the potential to reduce some of this confusion are displayed in Figure 1.
Two accessories that are used with this tubing, but that the healthcare provider would purchase separately, are a flushing pump and a water bottle (see: Figure 2 — which is only available in the more complete PDF version of this article — click here for a copy).
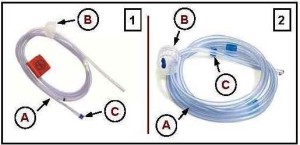
Like the reusable auxiliary water system marketed by Olympus America (and comparable ones marketed by other manufacturers), disposable tubing is intended for the irrigation of colonic and gastric mucosa via the GI endoscope’s auxiliary water channel,[2] although some manufacturers of this tubing may advertise its use, with a single-use adapter, for irrigation via the wider working channel if, for example, the GI endoscope is without an auxiliary water channel.CC
[Figure 1’s caption: Disposable irrigation tubing marketed by two manufacturers for use during GI endoscopy are displayed. The tubing (“A”) features a threaded cap (“B”) (with a vent for air displacement) for a secure connection to a water bottle. Embedded into the tubing’s other end is a permanent (primary) one-way valve (“C”; also see: Figure 3). An endoscope connector, which is not displayed in this figure—but three brands of which are displayed in Figure 4—is used to attach this tubing’s end to the GI endoscope’s auxiliary water channel via a port. According to some manufacturers, this tubing (“A”) may also be used with an single-use adapter that may connect to the GI endoscope’s biopsy port to achieve enhanced irrigation via the wider working channel. The endoscope connector may be single-use or reusable, and if the former, is typically designed with its own (secondary) one-way valve. Activation of a flushing pump (see: Figure 2) causes water from the water bottle to flow through this tubing (“A”) for irrigation of the GI tract’s mucosa. (These two images are printed with the expressed permission of their respective manufacturer.)]
But, unlike the reprocessing requirements of the reusable auxiliary water system’s reusable AWT, disposable irrigation tubing is not reprocessed.[4]
Suggesting that this disposable tubing may be easier and safer to use (than the reusable AWT, which requires reprocessing after each use[2]), at least two of its manufacturers issued statements that singled-out the Murfreesboro VAMC’s and Miami VAMC’s breaches as validation that reusable irrigation tubing can become contaminated with blood and be improperly reprocessed.[5,6]
Note: Three companies that market disposable irrigation tubing—Byrne Medical, now Medivators; ERBE USA; and U.S. Endoscopy (now a subsidiary of the STERIS Corporation)—participated in this review, providing Dr. Muscarella, this article’s author, with samples and/or photographs of their disposable irrigation tubing and endoscope connectors. Each also reviewed a draft of this article.

DISCUSSION
This article discusses the features, design, labeling and common uses of disposable irrigation tubing used during GI endoscopy. For the readers convenience, this discussion, which is sectionalized, is summarized in Table 3 (embedded on the right).
Guidance for the safe use of this type of tubing is featured in a future article, which is now available by clicking this link.
Whether disposable irrigation tubing may provide a safe alternative to reusable auxiliary water systems warrants consideration and discussion.
SECTION 1—Common features
Several companies market disposable irrigation tubing and its accessories to GI endoscopy departments. An alternative to reusable auxiliary water systems (such as Olympus America’s) for irrigation via the GI endoscope’s auxiliary water channel,DD disposable irrigation tubing is relatively simple to setup and use, and those marketed by different manufacturers share a number of features in common. Several of these features are listed in Table 2.
For instance, this tubing is used with a flushing pump and a water bottle (see: Figure 2). (Both Table 2 and Figure 2 are only available in the complete PDF version of this article — click here to download a copy).
Further, whereas near one of its two ends is a threaded cap that is designed to fit securely onto most types of disposable water bottles, embedded into the other end of virtually every manufacturer’s disposable irrigation tubing is a one-way (primary) valveEE intended, in part, to prevent the tubing’s contamination due to the backflow of potentially infectious materials and fluids during GI endoscopy (see: Figure 3, which is not included in this post, but is available in the complete PDF version of this article — click here for a copy).[1]
This valve is also designed to prevent water from leaking from the irrigation tubing whenever this tubing is disconnected from the GI endoscope (e.g., at the end of the endoscopic exam). (Note: Some earlier designs of this type of tubing may not have included this one-way back-flow valve.)
Case Reviews for Patients, Hospitals, Manufacturers: Click here to read about Dr. Muscarella’s expertise and legal assessments of the causes of healthcare-associated infections, including “superbug” outbreaks linked to contaminated GI endoscopes and other reusable medical equipment.
This type of tubing is universally marketed as “disposable.”
By virtue of this moniker, therefore, this tubing—most certainly, unlike the reusable auxiliary water system’s AWT, which requires reprocessing after each GI endoscopic procedure—is not designed (or labeled) to be reprocessed.
While its uses, labeling and designs are similar, however, there are a few features that may differentiate one manufacturer’s disposable irrigation tubing (used during GI endoscopy) from another’s (see: Section 4, below).
The endoscope connector
Manufacturers of disposable irrigation tubing generally also sell an array of endoscope connectors—which, as their name suggests, connect this tubing to the auxiliary water channel of different models and brands of GI endoscopes (via a port).
These connectors are purchased separately from, and are sold as accessories to, the tubing.
Depending on the manufacturer, the packaging of these connectors may be labeledFF for single-patient use or for 24-hour use (i.e., reuse on patients) sans reprocessing.
Virtually all disposable irrigation tubing features an embedded (primary) one-way “back-flow” valve. — Lawrence F Muscarella PhD
Moreover, whereas virtually all single-use endoscope connectors feature a one-way (secondary) valve to reduce the risk of contamination of the tubing, the tubing’s embedded (primary) one-way valve, and the water bottle with potentially infectious patient materials and fluids (see: Figure 4),[7] endoscope connectors promotedGG for reuse on multiple patients throughout the day (sans reprocessing), however, are typically designed without a one-way valve (again, depending on the tubing’s manufacturer).[8,9]
But, on to these reusable connectors users may manually attach a separately purchased single-use, one-way valve that is removed and discarded after each GI endoscopic procedure.[8,9]
Attention: Like most of this article’s tables and figures, Figure 4 is only available in the complete PDF version of this article, which does not include any advertisements — click here to download a copy.
No doubt, the endoscope connector’s design and labeling are important considerations.
Not only whether it is single-use or reusable, but also whether the endoscope connector is manufactured (or manually fitted) with a one-way (secondary) valve can impact the irrigation tubing’s safety, quality and effectiveness.
Similarly, whether this tubing is used instead with a single-use adapter that provides for irrigation via the GI endoscope’s working channel can, too, have important safety and infection control implications.HH
Moreover, reusable endoscope connectors do not have to be purchased exclusively from the irrigation tubing’s manufacturer.
The manufacturer of the GI endoscope (i.e., Olympus America, Pentax and FujiFilm), for example, generally equips its models, featuring an auxiliary water channel, with an endoscope connector that, if compatible, may be used with this tubing.
Because this endoscope connector is reusable, however, unlike disposable tubing, it requires reprocessing.
Note: While anecdotal, correspondence with stakeholders suggest that the Food and Drug Administration (FDA) might require, sometime in the future, that the endoscope connectors sold by the manufacturers of disposable irrigation tubing: (i) be labeled (and marketed) as single-use items; and (ii) feature a one-way “backflow” valve.[5,7,10]
SECTION 2—Advertised uses
Table 4, which is only available in the complete PDF version of this article — click here to download a copy, lists a number of the claims and advertised uses associated with disposable irrigation tubing.
This tubing’s “disposable” tag notwithstanding, these advertised claims include using this tubing “for a day” and “then throw it away” and, commonly, for “24-hour use,” which may be construed by the healthcare provider as these claims suggesting that this tubing was cleared by the FDA for reuse throughout the day.
This promoted application is notable because, as previously stated, the manufacturers of this irrigation tubing contraindicate its reprocessing.
Whether or not the endoscope connector features a one-way valve can impact the tubing’s quality. — Lawrence F Muscarella PhD
Like reusable auxiliary water systems, however, the use of disposable irrigation tubing may be associated with its own type of confusion. Box A discusses some questions that may arise from the marketing of this tubing—for example:
How do the manufacturers of this tubing define “disposable”?
Also discussed in Box A, while one of this tubing’s selling points is that, by eliminating reprocessing, it can reduce confusion and the risk of user error (see: Table 4),[2,5,6] precisely how the FDA originally intended this tubing to be marketed and used is unclear.
The reuse of some disposable items, like hypodermic needles, syringes, and single-patient medicine vials, is most certainly contraindicated because of this practice’s significant risk of infection (see: Box A).
(Note: Like most of this article’s tables, figures, and box article, Box A is only available in the complete PDF version of this article, which is without advertisements — click here to download a copy.)
SECTION 3A—Labeling – A device’s indications for use
A centerpiece of any 510(k) application, which the manufacturer submits to the FDA prior to marketing a device, is, as much as a description of the device, the device’s “indications for use.” Formatted as a statement that is featured in a section of the device’s submitted labeling, the indications for use include, for example, the device’s specific purpose and its target population.[11]
The FDA reviews this submitted application, and if it determines that the device is “substantially equivalent” to another legally marketed device, known as the “predicate” device,JJ the FDA then “clears” the device by way of a letter,KK granting the manufacturer the legal right to market the device in the U.S.
The device’s indications-for-use statement, along with its other labeling—for example, the device’s instructions for use, or “IFUs”—have important legal implications, defining for the healthcare provider the device’s “on-label” (i.e., “correct”) use, as opposed to the device’s “off-label” use.LL
Despite their “disposable” tag, irrigation tubing is usually marketed for “24-hour use,” which understandably may be interpreted by the user to be synonymous with a “multiple patient use” claim. — Lawrence F Muscarella PhD
Ordinarily, the FDA requires that the contents of the device’s cleared application (e.g., the device’s indications-for-use statement) necessarily be consistent with all of the device’s published labeling, advertised claims, and IFUs (instructions for use), because this documentation specifies important details for the healthcare provider—for example, whether the device is reusable or a single-use item.
In general, if a device’s labeling does not provide reprocessing instructions, then, by default, the device is a single-use device that is not to be reused or shared among patients.[12]
The off-label use of a medical device, in contrast, is ordinarily one for which the FDA did not specifically clear the device (e.g., the use is not included in the device’s indications-for-use statement),[11] and, therefore, while permissible and at times an acceptable medical standard,[11] a device’s off-label use may (but does not necessarily) increase legal exposure and the risk of patient harm, shifting liability (in part or totality) from the device’s manufacturer to the user, which could be a concern if an instance of patient injury (e.g., disease transmission) were linked to the device.[7]
As one manufacturer aptly writes in the IFU of its irrigation tubing’s single-use endoscope connector:
“This disposable medical device is not intended for reuse. Any institution, practitioner, or third party who reprocesses, refurbishes, remanufactures, resterilizes, and/or reuses this disposable medical device must bear full responsibility for their safety and effectiveness.”[7]
The healthcare provider’s clear understanding of a device’s on-label use, therefore, has legal implications and is important to the device’s safe and effective use.MM
Like reusable water systems, the use of disposable tubing is not entirely without confusion. — Lawrence F Muscarella PhD
SECTION 3B—Labeling – This tubings’ FDA clearances
A study of disposable irrigation tubing would be incomplete, therefore, if the content of its 510(k) clearances—which provides important information about the tubing’s safe and effective use—was not reviewed.
Bringing into clearer focus those practices that would define the on-label uses of disposable irrigation tubing, like those of any device, Table 5 lists the FDA’s 510(k) clearances of several of the irrigation tubing currently marketed in the U.S. Although a device’s advertised claims are ordinarily, if necessarily, consistent with its cleared indications for use, a comparison of Table 4 with Table 5 suggests some potential incongruities between this tubing’s marketed uses and its FDA-cleared labeling.
Attention: Like most of this article’s tables and figures, Table 4 and Table 5 are only available in the complete PDF version of this article, which does not include any advertisements — click here to download a copy.
Displayed in Table 5, related tubing that delivers sterile water from a water bottle – not to its auxiliary water channel – but to the GI endoscope’s air/water channels was cleared by the FDA in 1997 for “single patient use” (refer to FDA clearance number: K971125). Similar tubing with the same intended use was cleared again in 2009 (refer to FDA clearance number: K093665).
A study of disposable irrigation tubing would be incomplete if the details and content of its 510(k) clearances were not reviewed and discussed. — Lawrence F Muscarella PhD
To be distinguished from these two clearances, Table 5 shows that disposable tubing intended for irrigation specifically via the GI endoscope’s auxiliary water channel—this type of tubing is the focus of this article—was first cleared by the FDA in 2003, also for “single patient use” (refer to FDA clearance number: K031773).
Listed in Table 5, irrigation tubing for this same application was cleared by the FDA, for the second time, in 2009 (refer to FDA clearance number: K092429)
Both of these clearances describe irrigation tubing that either has a single-use claim or uses as its predicate device tubing with a single-use claim.
Like each of the tubing that the FDA subsequently cleared, however, the documentation of this latter tubing (cleared in 2009), including the indications-for-use statement, does not state whether the tubing is intended for single-patient use or reuse (the importance of such clarification notwithstanding).
Summarizing Table 5, this type of tubing (originally intended for irrigation via the GI endoscope’s auxiliary water channel), to date, has been cleared by the FDA, either directly or indirectly via a predicate device, with a single-use claim—namely:
- as tubing cleared for single-patient use only (i.e., K031773);
- as tubing (i.e., K092429) that (lacking the number of patients in its clearance on whom the device is intended) uses, as its predicate device, this latter tubing cleared in 2003 (i.e., K031773), which has a single-patient-use claim; or
- as tubing (e.g., K103239) that (also lacking the number of patients in its clearance on whom the device is intended) uses, as its predicate device, irrigation tubing (i.e., K092429) whose own respective predicate device is tubing cleared by the FDA for single-patient use (i.e., K031773).
Suggesting further the possibility that the FDA may have originally intended disposable irrigation tubing to be a single-use item, this review did not identify any such tubing, including those listed in Table 5, that was cleared by the FDA specifically for reuse on more than one patient (some of Table 4’s advertised clams notwithstanding).
That said, instances of disease transmission linked to the reuse of this disposable irrigation tubing have not been reported during GI endoscopy. — Lawrence F Muscarella, PhD
Three notable observations
Three notable observations arise from this review of the FDA’s clearances of irrigation tubing (see: Table 5):
First, while the reuse of “disposable” irrigation tubing on multiple patients during a 24-hour time frame (sans reprocessing) is not described in these tubings’ FDA-cleared applications (and, therefore, appears to be an off-label use), cases linking this tubing’s reuse (consistent with the tubing’s advertised claims; see: Table 4) to specific instances of disease transmission (or another type of patient harm) during GI endoscopy have not been reported.
Second, it is only reasonable to conclude that the FDA is aware that this disposable irrigation tubing is marketed for reuse on multiple patients during a 24-hour time frame. Indeed, claims advertising (or implying) that this tubing can be reused have been available for some time both on manufacturers’ websites and in product brochures.[5,8,9,13-15]
And, having seemingly not demurred, the FDA appears to have tacitly approbated the reuse of this tubing (but not necessarily of the endoscope connector[7,10]), the tubing’s “disposable” tag and clearances notwithstanding (see: Table 5).
And, third, considered in Box B — which, like this article’s other box articles, is only available in the complete PDF version of this article, which can be downloaded by clicking here — is whether there may be precedents that rationalize the reuse of “disposable” irrigation tubing—like the “disposable” water bottle to which this tubing may be connected.
Nevertheless, clarification of whether the FDA has indeed concluded that this tubing’s reuse is safe, like the FDA’s definition of “disposable” vis-à-vis this irrigation tubing, is respectfully requested.
SECTION 4— Irrigation tubing differences
The features, design, and labeling of different brands of disposable irrigation tubing and its accessories are not all alike.
For example, one tubing manufacturer’s endoscope connector may be constructed primarily of plastic, whereas another manufacturer’s connector may contain more durable metal (which is intended to perform better and to be less prone to water leakage).[7,8]
Some other differences between the tubing are provided in Box C, which, like Box A, is only available in the complete PDF version of this article, which can be downloaded by clicking here.
CONCLUSIONS
This study of disposable irrigation tubing raises some interesting questions about the marketing, clearances, and uses of medical devices, as well as their regulation by the FDA.
Indeed, few other examples might display the FDA’s apparent countenancing of the reuse on multiple patients of a device whose clearances by the FDA are associated, either directly or via a predicate device, with a single-use claim (see: Table 5).
Not so much between the tubing, but the differences between the design, labeling, and marketing of the endoscope connector, and, too, whether the tubing might be used instead with an adapter for irrigation via the GI endoscope’s working channel, provide an opportunity both for circumspection and to enhance both quality and safety.
Note: Guidance for using this disposable irrigation tubing is featured in the 3rd article in this series — click here.
Providing some guidance, GI departments that are using—without trouble, inconvenience, or identified missteps—reusable auxiliary water systems and their tubing may be inclined to continue doing so, applying the adage that “if it isn’t broken, then don’t try to fit it.”
The use of these reusable systems is acceptable, of course, provided a number of criteria are satisfied—for example, that the reusable auxiliary water system’s AWT (and its accompanying endoscope connector) is reprocessed after each use.
GI endoscopy units that prefer to use disposable irrigation tubing, however, whose reprocessing its manufacturers contraindicate, may appreciate some of the conveniences it offers.
In closing, more detailed guidance for the safe use of disposable irrigation tubing (as well as of reusable auxiliary water systems) is featured in a subsequent article that can be read by clicking this link.
KEY: Key to article (for example, the text associated with the supra-script “AA“) is provided on the next page:
AA: Whereas this series of articles defines “disposable” items as those that are used on one patient and are then discarded (e.g., disposable biopsy forceps), it defines “reposable” items as those that are reused throughout the day on several patients and are then discarded (i.e., a single-day item), without reprocessing. The definitions of these two terms, along with that of a “reusable” item, are provided in Box B of the first article in this series, on p. 4.[1]
BB: The “disposable” tag associated with its marketing and advertising notwithstanding, whether this type of irrigation tubing is intended for use on a single patient or for daily reuse on multiple patients before being discarded warrants clarification.
CC: Although this article focuses on disposable irrigation tubing that connects to the auxiliary water channel of Olympus GI endoscopes, its discussions may also be applicable to:
- other manufacturers of GI endoscopes (e.g., Pentax, FujiFilm);
- other types of tubing and systems that may be similarly used for irrigation during GI endoscopy; and
- irrigation achieved using the GI endoscope’s working channel (which may be performed, for example, if the GI endoscope is without an auxiliary water channel).
DD: This review does not, per se, evaluate and compare the performance of disposable irrigation tubing sold by different manufacturers, although differences in this tubing—for example, the internal diameter of one manufacturer’s tubing may be narrower than another’s—could impact performance by affecting the flow of the water delivered into the GI tract during irrigation.
EE: The purpose of the tubing’s one-way valve is the same as that of the reusable auxiliary water tube’s (“AWT”), although the former’s valve is located in the end of the tubing that connects to the GI endoscope, whereas the AWT’s valve is located in the end of the tubing that connects to the short OFP irrigation tube (“SIT”), which is often several feet from the GI endoscope.[1]
FF: By “labeling,” this article refers to any of a manufacturer’s published materials that are associated with a device’s use (e.g., the device’s indications for use, the instructions on its packaging, or information about its use on the manufacturer’s website).
GG: By “promotion,” this article refers to any of a manufacturer’s written or stated claims, including those in the device’s labeling and on the manufacturer’s website, that support or encourage the device’s sale, marketing and/or use in a specific manner.
HH: This type of disposable tubing was originally cleared by the FDA for irrigation via the GI endoscope’s auxiliary water channel (refer to the FDA’s clearance: K031773). Nevertheless, this tubing may also be marketed for use with a single-use adapter for irrigation through the GI endoscope’s working channel, via the GI endoscope’s biopsy port, to provide for a greater volume of water during irrigation (the diameter of the working channel is wider than that of the auxiliary water channel), as may be required of a poorly prepped colon. (The author deliberately skipped the key “II.”)
JJ: Or, it could be cleared via a “de novo classification,” which provides a regulatory route for low- to moderate-risk devices that are not substantially equivalent to a legally marketed device.
KK: Available on-line, a device’s “510(k) clearance” letter clarifies the indications for use for which the FDA cleared the device. An example of the clearance letter of a related device, inclusive of the device’s indications-for-use statement, is available by clicking here.
LL: When solicited, a manufacturer may discuss off-label uses of a device (e.g., for the purpose of education), but it is legally precluded from promoting the device for its use in a manner for which the FDA did not expressly clear it.
MM: The clarity of a device’s labeling is paramount. Effective controls by the manufacturer that prevent the device’s labeling from being misleading or confusing—examples would include unsubstantiated claims or ambiguous labeling—are crucial to patient safety, as well as to ensure that the device does not become misbranded.
References: Click here.
Article by: Lawrence F Muscarella, PhD; posted: August 15, 2013; updated: 1/27/2015, Rev B.