May 5, 2016 — At least three patients died following an endoscopic procedure performed last year at Huntington Memorial Hospital in Pasadena (CA), the Los Angeles Times disclosed today in a breaking front-page news report.
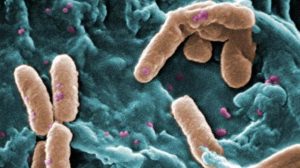
Each of the patients was infected with a multidrug-resistant Pseudomonas bacteria after undergoing ERCP.
Although this deadly outbreak was identified in August 2015, only now are hospital officials acknowledging the deaths of these three patients. These patients were previously thought only to have been infected with the bacteria, not necessarily to have expired.
ERCP — or “endoscopic retrograde cholangiopancreatography” — is an endoscopic procedure performed more than 650,000 times annually in U.S. hospitals using a duodenoscope. The procedure is routinely performed to diagnose and treat certain disorders and diseases of the biliary and pancreatic ductal systems.
The mortality rate of patients infected with multidrug-resistant bacteria — CRE is another example — can be as high as 50%. Both Pseudomonas and CRE can be found in soil, water, and a patient’s gastrointestinal tract.
Summer of 2015
Last year, on August 19, 2015, The Los Angeles Times reported that Huntington Memorial was investigating a suspected outbreak related to a contaminated duodenoscope –the same type of endoscope that has been linked since 2012 to more than a dozen other outbreaks of CRE — or “carbapenem-resistant Enterobacteriaceae” — and related superbugs.
Today’s Times report discloses for the first time what the hospital, state and local health officials have not previously revealed to the public about this outbreak: that the three infected patients died.
To date, the hospital has provided few details about these three patients, citing medical privacy laws, according to last year’s Times article.
Nor has Huntington Memorial publicly discussed how many additional patients might have been exposed to this same Pseudomonas superbug, or whether these “at risk” patients who underwent ERCP last summer were contacted and notified of the potential for infection.
A regulatory report
According to a regulatory report filed with the FDA last year on August 22, 2015 (this report provides some important details about Huntington Memorial’s deadly outbreak), the hospital’s three patients were infected with a multidrug-resistant Pseudomonas bacteria and diagnosed with “septicemia” following ERCP.
 These ERCP procedures were performed using an Olympus TJF-160F duodenoscope (Olympus America) — not the TJF-Q180V model.
These ERCP procedures were performed using an Olympus TJF-160F duodenoscope (Olympus America) — not the TJF-Q180V model.
One month later, Olympus filed a response to the hospital’s report, after it learned about the outbreak via a news article (apparently, not from the hospital).
According to Olympus’ filed response, “the (duodenoscope) has been cultured by the (hospital) but test results were not provided.” This claim raises the possibility that the hospital confirmed that the duodenoscope was the source of the deadly Pseudomonas outbreak (a finding that requires confirmation).
Recommended reading: Hospitals investigating measures to prevent deadly superbug outbreaks linked to ERCP can read my peer-reviewed article — “Risk of transmission of carbapenem-resistant Enterobacteriaceae (CRE) and related ‘superbugs’ during gastrointestinal endoscopy” (World Journal of Gastrointestinal Endoscopy) — which provides a number of recommendations.
Earlier this year, the FDA published a safety communication — “Olympus Validates Updated Reprocessing Instructions for Duodenoscope Models TJF-160F and TJF-160VF” — confirming that the TJF-160F model’s original reprocessing instructions had not been properly validated for safety until just recently, months after Huntington’s outbreak.
The FDA raised concerns last year about the newer, but related, Olympus TJF-Q180V duodenoscope, which hinders cleaning and may pose a risk of deadly infections following ERCP, according to the FDA.
An investigation published in January by Senator Patty Murray (D-WA) linked the Olympus TJF-Q180V duodenoscope to more than a dozen outbreaks in the U.S., including last year’s well-publicized deadly CRE outbreak at UCLA Ronald Reagan Medical Center, which infected 8 patients, at least two of whom died.
Olympus recalled the TJF-Q180V duodenoscope model in January amid concerns about it safety.
In addition to Olympus, duodenoscopes sold by Pentax (Montvale, NJ) and FujiFilm (Wayne, NJ) have been linked to bacterial outbreaks and federal warnings.
Today’s news about Huntington Memorial’s outbreak reveals that the Olympus TJF-160F duodenoscope, too, is prone to transmitting deadly infections.
Outbreak Highlights
- Huntington Memorial’s three infected patients expired.
- This outbreak’s ‘superbug’ was a multidrug-resistant Pseudomonas.
- This hospital’s outbreak was linked to the Olympus TJF-160F duodenoscope, which reveals that this model, too — not only the newer Olympus TJF-Q180V model, may hinder effective cleaning.
- The cleaning instructions of the Olympus TJF-160F duodenoscope were not validated until earlier this year, several months after Huntington Memorial’s outbreak.
- Hospital officials, along with state regulators and the city of Pasadena’s Public Health Department, appear not to have informed the families of the three expired patients that a contaminated duodenoscope could have contributed to this outbreak.
- Hospital and health officials also may not have notified (as a safety precaution) any of Huntington Memorial’s other “at risk” patients — possibly dozens, on whom the same potentially contaminated duodenoscope was used — that they, too, could have been exposed to, and either colonized or infected with, the outbreak’s bacteria.
Disputed information
As reported by ABC 7 News and CBS News last August, Huntington Memorial’s officials said that these three “patients … were very ill before they underwent (ERCP), and the risk of the procedure was explained to each patient and family.”
Last year’s Times article similarly quoted hospital officials to say, “Even though the link between the scope and bacteria is not confirmed, we alerted the affected patients about a possible link as well as reported the bacterial growth to health officials.”
But, whether this happened is in dispute.
An attorney representing at least two patients infected at Huntington Memorial last summer said that the families were not told important details about the outbreak — such as the duodenoscope’s possible role — by either the hospital or government health officials, including state regulators and the city of Pasadena’s Public Health Department.
According to this attorney, the hospital “told the families basically nothing.”
And, the death certificates of two of Huntington’s infected patients who died do not list multidrug-resistant Pseudomonas, a hospital-associated infection, or a potentially contaminated duodenoscope as possible causes of death — despite the regulatory reports the hospital and Olympus filed with the FDA suggesting the infection may have contributed to the deaths.
Notifiying patients of the risk
In 2013, Advocate Lutheran General Hospital (Park Ridge, IL) linked a similar deadly superbug outbreak to ERCP and, violating no federal HIPAA laws, made a “public plea” then asking that any patient who underwent ERCP at the time of the outbreak — and therefore might have been exposed to the highly drug-resistant bacteria — return for screening. More than three dozen of this hospital’s patients were found to be “positive” for the outbreak’s bacteria.
Last year in February, UCLA’s Ronald Reagan Hospital similarly informed nearly 180 patients who underwent ERCP of their potential exposure to a duodenoscope contaminated with a deadly superbug. UCLA had linked eight patients infected with CRE to contaminated duodenoscopes in early 2015. At least two of these infected patients died.
And Virginia Mason Medical Center (Seattle, WA), which encountered a deadly bacterial outbreak in 2012, notified dozens of potentially “at risk” patients. As many as 18 infected patients died.
It is unclear how many other patients of Huntington Memorial who underwent ERCP at the time of the outbreak may have been exposed to this bacteria, and whether they were informed of the risk.
For its part, Huntington officials told the Times last year that to prevent additional infections, the hospital had begun quarantining duodenoscopes and was not reusing them until cultures suggested the endoscopes were no longer contaminated with bacteria.
![]() Confidential Quality, Safety and Legal Reviews for Hospitals, Manufacturers and the Public: Click here to read about Dr. Muscarella’s quality and safety services committed to assisting manufacturers, hospitals and patients reduce the risk of healthcare-associated infections, including ‘superbug’ outbreaks linked to contaminated duodenoscopes.
Confidential Quality, Safety and Legal Reviews for Hospitals, Manufacturers and the Public: Click here to read about Dr. Muscarella’s quality and safety services committed to assisting manufacturers, hospitals and patients reduce the risk of healthcare-associated infections, including ‘superbug’ outbreaks linked to contaminated duodenoscopes.
Another deadly superbug outbreak
Last February, I linked the Olympus TJF-Q180V model to an outbreak that infected eight patients at a U.S. hospital with a deadly strain of E. coli just a few weeks earlier. Two of these patients died, but the location of the outbreak remains a mystery, as no hospital has publicly acknowledged these eight infections.
This deadly E. coli outbreak may be this year’s most significant bacterial outbreak linked to a duodenoscope. This outbreak was also discussed in both The Los Angeles Times and the New York Post.
Once more is learned about it, this E. coli outbreak likely will share some of the traits of Huntington Memorial’s Pseudomonas outbreak.
The TJF-160F vs. the TJF-Q180V Olympus duodenoscope models
Briefly, the Olympus TJF-160F is an older duodenoscope model whose instructions require healthcare personnel to clean and disinfect — a process known as “reprocessing” — a small narrow channel called an elevator wire channel (as well as all of the endoscope’s other channels and surfaces).
The elevator wire channel houses a narrow cable that connects to a forceps elevator mechanism at the duodenoscope’s distal tip. Doctors use this mechanism and cable, which is attached to a control knob on the endoscope’s head, to manipulate the direction of needles, cannulae and other accessories used during ERCP.
The elevator wire channel of the newer TJF-Q180V duodenoscope model, in contrast, is “sealed” (although this seal may be worn and leak), in part for convenience, so that reprocessing personnel no longer have to flush this channel manually with detergent and disinfectant. According to multiple reports, the design of this endoscope model and sealed channel is flawed, posing a increased risk of patient infection.
Commentary: Is silence the new norm?
The news reported today that Huntington Memorial’s three infected patients died last year displays a notification delay of several months, which, while less than ideal, is not uncommon.
As I wrote in a previously article, “silence” appears to have become the new norm, with important details about a hospital’s deadly outbreak not being publicly revealed until months — and in some cases — years later.
Public disclosure and the sharing with other hospitals, less of the fine details of a hospital’s outbreak, than of the actions the hospital adopted in response to the outbreak has been a mainstay to public health, is crucial to the integrity of healthcare, and necessary to the prevention of additional potentially deadly infections.
While I do not endorse hospitals practicing such silence, federal, state and local health authorities are held to an even higher standard. The public expects these authorities to inform it promptly of an identified outbreak and its associated deaths, among other reasons, to create competition; improve safety, transparency, and trust; reduce costs; appear objective; and prevent the appearance that they are being influenced by hospitals, to the detriment of public health.
Transparency improves safety. It’s the best disinfectant available.
 Article by: Lawrence F Muscarella, PhD. Posted 05/05/2016; revised: Rev C. LFM Healthcare Solutions, LLC. All rights reserved.
Article by: Lawrence F Muscarella, PhD. Posted 05/05/2016; revised: Rev C. LFM Healthcare Solutions, LLC. All rights reserved.
E: Larry@LFM-HCS.com. Twitter: @MuskiePhD
