October 1, 2018 (updated: Oct. 5, 2018; edited: Oct. 8, 2020) — A bronchoscope’s use has been linked again to a “superbug” outbreak, this time involving “multiple patients,” according to a regulatory report filed 4 months ago.
The report provides few additional details, and it does not identify the specific number of patients infected, or whether any patients expired. The bronchoscope’s manufacturer reported these infections to the FDA on May 31st.
Nor does the report identify the species responsible for this outbreak other than that the organism is an “unspecified” strain of the feared “CRE” superbug, known more formally as carbapenem-resistant Enterobacteriaceae. The model identified in the report is the BF-P190 bronchoscope.
CRE can spread from person-to-person, and their infections are potentially deadly. According to the U.S. Centers for Disease Control and Prevention (CDC), the mortality rate associated with this superbug’s infections can be as high as 50%.
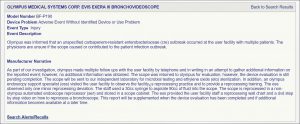
The cause of the infections described in this May regulatory report is unknown, and the facility’s physicians are unsure if the bronchoscope “caused or contributed to the patient infection outbreak,”[*] the regulatory report states.
A portion of the report is provided to the right. It states that a manufacturer representative visited the medical facility in response to the infections and identified only “one minor” reprocessing lapse. (The name of the U.S. medical facility is redacted.)
Federal alerts and safety communications publicizing the risk of bronchoscopes potentially exposing patients to CRE or a related multidrug-resistant organism (“MDRO”) are scant.

However, the FDA and CDC have advised the public about the public health concerns of duodenoscopes, which are another type of flexible endoscope, infecting patients with CRE and related superbugs.
In February 2015, the FDA issued the first of several alerts publicizing the emerging risk of these complex devices exposing patients to multidrug-resistant bacteria, including CRE. Duodenoscopes, unlike simpler bronchoscopes, feature a forceps elevator mechanism, at their distal end, whose moving parts can be difficult to access and clean with a brush.
In response, the FDA has advised that thoroughly cleaning duodenoscopes “prior to high-level disinfection should reduce the risk of transmitting infection, but may not entirely eliminate it.”
Later in 2015, the agency issued a second alert again to publicize this safety risk, reporting that the “unique and complex design” of the duodenoscope “presents challenges for effective reprocessing.” The alert suggested that facilities using duodenoscopes consider adopting one or more additional measures, such as sterilization, to reduce the infection risk.
These alerts focusing on the designs of duodenoscopes marked a watershed moment because almost all of the cases of cross-infection linked the previous 20 years to a contaminated endoscope were due to a cleaning or disinfection lapse, not to a thoroughly cleaned and high-level disinfected endoscope.
Reprocessing, or the proper cleaning and disinfection (or sterilization) of flexible endoscopes, is a multi-step process crucial to prevent the transmission of potentially life-threatening infections during the procedure.
Since 2012, more than two dozen outbreaks of CRE and related multidrug-resistant bacteria have been linked to duodenoscopes. Klebsiella species and E. coli are two of the types of CRE that have been recovered from duodenoscopes. These same superbugs, along with other related strains of carbapenem-resistant bacteria, may also contaminate bronchoscopes and other types of flexible endoscopes, including gastroscopes and urologic endoscopes during the procedure.
CRE and related superbugs, such as carbapenem-resistant Pseudomonas aeruginosa, are resistant to several antibiotics including carbapenems, which are a “last defense” against serious healthcare-associated infections. Colistin may be the only remaining antibiotic effective against CRE infections.
Bronchoscopes are used about 500,000 times a year in the U.S by lung specialists to diagnose and treat a variety of lung- and airway-related diseases in patients. Duodenoscopes are used a similarly number of times a year in the U.S. to examine and treat diseases of the pancreatic and bile ducts.
 Case Reviews, Expert Advice, Consulting Services: LFM-Healthcare Solutions, LLC provides medical expertise for hospitals, manufacturers and the public, specializing in healthcare-associated infections linked to medical equipment. Services include litigation support. Years of experience. Reasonable rates.
Case Reviews, Expert Advice, Consulting Services: LFM-Healthcare Solutions, LLC provides medical expertise for hospitals, manufacturers and the public, specializing in healthcare-associated infections linked to medical equipment. Services include litigation support. Years of experience. Reasonable rates.
A CRE cluster in 2014
This regulatory report filed in May (2018) was not the first submitted to the FDA identifying a possible association between bronchoscopy and CRE infections.
Four years earlier, a regulatory report linked the use of a bronchoscope to 14 patients testing positive for CRE and a susceptible strain of P. aeruginosa.[*] This 2014 report may document the first time the FDA was informed of the association between the use of a bronchoscope and the possible transmission of CRE.

A portion of this December regulatory report, which the FDA received on December 24, 2014, is provided to the right. (The name of this U.S. medical facility where this incident occurred is redacted.) The model identified in the report is the BF-160 bronchoscope.
The bronchoscope “repeatedly tested positive for cultures after reprocessing” and, the report adds, had been repaired by “a third-party vendor.”
Whether any patients expired is not identified in the report. According to the manufacturer, the “exact cause” of this incident “could not be conclusively determined at this time.”
The manufacturer of the bronchoscope explains that “use of a third party components on this scope may compromise the device and could result in harboring bacteria if the inside of the device becomes damaged.”
Also according to this manufacturer, the reprocessing procedures and protocol are “only validated for devices that meet (the manufacturer’s) specifications. The device is not validated when it serviced/repaired by a third party vendor.”
This December report’s case is discussed in more detail in the article, “A Bronchoscope Was Linked to a ‘Superbug’ Outbreak in 2014. CRE outbreaks linked to bronchoscopes may be under-recognized.”
Several bronchoscope models recalled
Last week, the manufacturer voluntarily recalled more than two dozen bronchoscope models in the U.S. due to a potential concern involving the device’s instrument channel port, or biopsy port. The recall was not limited to devices in the U.S. but also included Canada and other countries.
These several models were recalled on September 29th because “the attachment of non-Olympus accessories to the bronchoscope’s instrument channel port may have resulted in more applied force than expected and lead to loosening of the instrument channel port.”
The recall notices advise customers to inspect the affected bronchoscopes in accordance with the recall’s instructions, required actions and included diagrams. The device is not to be used and is to be returned to the manufacturer for repair if either the instrument channel port can be manually rotated or turned (as shown in a diagram included in the recall notices), or if the rubber part around this port “is lifted from the molding parts” (as shown in another provided diagram), these recall notices further advise.
However, if this port is not loose and this “rubber part is in a normal condition” (as displayed in a third included diagram), then the bronchoscope can be used in a patient procedure, according to the recall notices.
In a letter advising health care practitioners about this bronchoscope recall, the manufacturer stated it had “received a complaint claiming that patients were infected after a bronchoscopy procedure in which a BF-1T180 bronchoscope with a loosened instrument channel port was used.”
The BF-1T180 bronchoscope was one of the several models the manufacturer voluntarily recalled last week due to this issue with the instrument channel port. Included in this recall was the BF-160 model (but not the BF-P190 bronchoscope).
FDA reports of infections
The FDA’s adverse device database was reviewed and, consistent with the manufacturer’s letter discussing last week’s recalls, regulatory reports were identified describing the culturing of potentially infectious debris around the instrument channel port of the BF-1T180 model.
Four years earlier, for instance, the manufacturer notified the FDA on August 28, 2014, that “22 patients had tested positive for E. coli and Pseudomonas aeruginosa, and Candida after undergoing a bronchoscopy” the same year. All of the patients were reported to have been examined using the same bronchoscope, the filing states.[*]
The facility “conducted two sets of culture test on the o-ring of the device,” finding both culture tests to be positive for microorganisms, according to this 2014 report. (This o-ring may be the rubber part around the instrument channel port discussed in last week’s recall notices.)
This case’s filed regulatory reports do not state whether the bacteria and fungus were susceptible to antibiotics or multidrug resistant. Many of the patients received intravenous antibiotics, and all fully recovered, the reports state.
By way of another instance, a healthcare facility notified the FDA two years later, on October 11, 2016, that a “[r]eview of facility data reflects a recent trend of increased diagnosis of Pseudomonas aeruginosa in (patients) with bronchoscopy.” The report states that the bronchoscope was “was found to have a defective port that was moving (is supposed to be stationary) and the o-ring was displaced allowing bio-matter to accumulate.”[*] (This description was provided by the facility; an understanding of the manufacturer’s findings and assessment too are important for context.)
The next month, the manufacturer submitted reports to the FDA linking the bronchoscope to 13 patients who tested positive for P. aeruginosa. According to the reports dated November 3, 2016, “[a]pprox 2 out of the 13 patients were identified as having comorbidity medical conditions and have since expired.”[*]
Also describing finding biomatter under the bronchoscope’s o-ring, the reports state that “biomatter (was) found underneath the o-ring of the scope,” adding that the bronchoscope tested positive for the Pseudomonas after reprocessing.
The reports discussing this incident state that the “cause of the reported positive culture could not be determined but improper maintenance of the device cannot be ruled out as a contributory factor.” The reports do not identify whether the cluster’s Pseudomonas was susceptible to antibiotics or multidrug resistant.
Providing additional supplemental information, the manufacturer states in the reports performing a root cause analysis of this case and other similar complaints “related to loose biopsy ports.” According to the manufacturer, this analysis determined that “the biopsy port (k-port) will not become loose during normal use,” but that use of an incompatible accessory or equipment could possibly result in excessive torque forces, “resulting in loosening of the biopsy port.”
Other regulatory reports similarly describe identifying a loose cap or biopsy port on a bronchoscope with the area culturing positive for bacteria without identifying a lapse in the reprocessing procedure. Among other factors, a faulty device design or manufacturing error, lacking maintenance or an improper repair can cause a bronchoscope’s instrument channel port to become loose.
On December 27, 2017, the manufacturer submitted a regulatory report to the FDA linking a possible bacterial infection to the BF-1T160 bronchoscope model, which was also recalled last week due to this same issue with the instrument channel port.
According to this report, “during reprocessing, the bronchoscope was found with debris under the biopsy port and the biopsy port was found loose.” A manufacturer representative visited the facility and identified no reprocessing lapses, the report adds.
The manufacturer explains that the “cause of the reported event could not be conclusively determined; however, improper maintenance could not be ruled out as a contributing factor to the reported event,” adding in the report that the bronchoscope is serviced by a third party (not the device’s manufacturer).
Other bronchoscope components, too, may become loose and pose an infection risk. Another bronchoscope manufacturer recalled several models in June 9, 2017, “to retrospectively document the actions that were taken by (the company) to correct the loose suction arms on eleven (11) models of endoscopes and to notify FDA of these actions taken in 2010-2011.”
An accompanying field correction letter in the U.S. dated five weeks later on July 19, 2017, notified customers of this potential issue associated with the several bronchoscope models (purchased prior to January 26, 2011 ). According to the letter, a loose suction arm could pose a risk of organic debris accumulating “in the space between the suction nipple and control body” of the bronchoscope, and that in some cases “these events could potentially cause cross-contamination between patients.”
Bronchoscopes are not the only type of flexible endoscope whose design includes an o-ring that has been linked to infections of P. aeruginosa. Investigated an 2012 outbreak of carbapenem-resistant P. aeruginosa linked to a duodenoscope model, Verfaillie et al. (2015) suggested that the device’s o-ring “did not function properly and that adequate sealing of the elevator wire channel could not be guaranteed.”
 Case Reviews, Expert Safety Services: Click here to read about Dr. Muscarella’s quality and safety services designed to help clients reduce the risk of healthcare-associated infections, including superbug outbreaks linked to contaminated duodenoscopes and other types of reusable medical equipment.
Case Reviews, Expert Safety Services: Click here to read about Dr. Muscarella’s quality and safety services designed to help clients reduce the risk of healthcare-associated infections, including superbug outbreaks linked to contaminated duodenoscopes and other types of reusable medical equipment.
Earlier reports discussing a bronchoscope’s loose biopsy-port cap
Almost 15 years ago, investigators reported the contamination of bronchoscopes with bacteria due, at least in part, to a loose “biopsy-port cap.”
A study published in the New England Journal of Medicine (NEJM) in 2003 documented the bacterial contamination of bronchoscopes and possible infection of patients at a medical facility in Tennessee “as a result of the inadequate disinfection of bronchoscopes because of a manufacturing defect.”
The investigators found that the cap of the biopsy port of two bronchoscopes “were not securely fastened and were easily removable, contrary to manufacturing specifications. The port caps were loose enough to be unscrewed with two fingers but not so loose that the problem was apparent to the bronchoscopy staff.“
“When the threads of the biopsy ports and the inside of the caps of (the two bronchoscopes) were swabbed,” these investigators reported that “a dark green film was noted, and 9 of 12 swab specimens were positive for P. aeruginosa and S. marcescens. The other three swab specimens were positive for P. aeruginosa alone, including one specimen obtained after three (automated) cycles of cleaning and disinfection.”
A second study published in the same issue of NEJM reported an outbreak of P. aeruginosa infections in Maryland associated with bronchoscopy. The study’s authors reported that the infections were “apparently caused by a loose biopsy-port cap in the bronchoscopes,” which may have “sheltered organisms and thus rendered disinfection procedures ineffective.”
The study concluded that instrument safety and surveillance methods for bronchoscopy “must be improved, and better recall procedures are needed for medical devices.”
Notably, these studies published in 2003 are discussed herein only for historical purposes. The quality issues discussed in these two studies may be different from, and entirely unrelated to, the reason for last week’s recalls of several bronchoscope models.
FDA issues an alert in September 2015 about bronchoscopes
The FDA issued a safety notice almost five years ago, on September 17, 2015, entitled, “Infections Associated with Reprocessed Flexible Bronchoscopes.” A portion of this notice is provided to the right.
 While this alert did not specifically publicize that bronchoscopes may pose a risk of transmitting CRE and related superbugs, it did advise that a “small number” of regulatory reports submitted in 2014 indicate “persistent” contamination of the bronchoscope “despite following the manufacturer’s reprocessing instructions,” as the FDA had similarly reported could occur with duodenoscopes.
While this alert did not specifically publicize that bronchoscopes may pose a risk of transmitting CRE and related superbugs, it did advise that a “small number” of regulatory reports submitted in 2014 indicate “persistent” contamination of the bronchoscope “despite following the manufacturer’s reprocessing instructions,” as the FDA had similarly reported could occur with duodenoscopes.
Alipour et al. (2017) reported last year that standard cleaning and high-level disinfection of bronchoscopes may not be effective “against biofilm embedded forms.” These authors noted that bronchoscopes can also transmit colistin-resistant bacteria.
Factors that can contribute to a reprocessed bronchoscope remaining persistently contaminated and transmitting bacteria include “[c]ontinued use of devices despite integrity, maintenance and mechanical issues.”
Recommendations to improve bronchoscope safety
The following recommendations are provided to help facilities improve the safety of bronchoscopes and prevent the transmission of CRE and related superbugs:
- Review the manufacturer’s recall notices discussing last week’s recall of several bronchoscope models, and ensure compliance with the provided instructions.
- Consider the merit of adopting and applying to bronchoscopes (when deemed feasible and warranted — for example, in an outbreak setting) sterilization or one of the other supplemental measures the FDA published on August 4, 2015, to improve the effectiveness of duodenoscope reprocessing.
- Facilities may also consider (when available, appropriate and warranted) use of sterile instrumentation (e.g., a single-use flexible endoscope) or a FDA-cleared sterile sheath to cover the endoscope to reduce the risk of transmitting CRE or a related MDRO during an endoscopic procedure.
- Review the recommendations to improve bronchoscope safety that the FDA provided in the safety notice entitled, “Infections Associated with Reprocessed Flexible Bronchoscopes.” For example: “Follow the manufacturer’s recommendations for preventive maintenance and repair of the device.”
- Consider other preventive measures, too, including use of a borescope to examine the bronchoscope’s working channel for cleanliness as well as for abnormalities or irregularities that could pose an infection risk — for example, tears, scratches, perforations and other damage, and the presence of residual soils and blood.
This list of recommendations is incomplete. Review the manufacturer’s instructions and device labeling for additional safety guidance.
Conclusion
Published data now suggest that bronchoscopes pose a risk of exposing patients to CRE and related MDROs. Enhanced safety measures and reprocessing practices may be considered (depending on the clinical circumstances — for example, in an outbreak setting) to improve safety.
[*] Footnote: (i) “Linking” (or “associating”) an endoscope or another type of reusable device to (or with) an infection or an outbreak does not confirm the device necessarily transmitted and caused the infection. More data would be required to conclude more definitively a causal relationship between the device and the infection. (ii) Regulatory reports submitted to the FDA routinely describe important clinical cases and experiences, but the findings and conclusions of these reports generally have not been peer-reviewed and may be incomplete, or, at least in part, inaccurate.
Article by: Lawrence F Muscarella, PhD. Posted Oct. 1, 2018; updated Oct. 5, 2018; edited Oct. 6, 2020. Copyright (2018). LFM Healthcare Solutions, LLC. All rights reserved. Dr. Muscarella is the president of LFM Healthcare Solutions, LLC, an independent quality improvement company. Click here for a discussion of his quality improvement healthcare services.
E: Larry@LFM-HCS.com. Twitter: @MuskiePhD
Thanks for writing this article—scary!